american academy of pediatrics covid vaccine 12-15
The COVID vaccine is now available for children ages 1215. Type 1 and Type 2 American Diabetes Association.

Pfizer Wants Authorization To Give Covid 19 Vaccine To Kids Over 12
The American Academy of Pediatrics states that most childhood vaccines are 90-99 effective in preventing disease According to ShotLife a United Nations Foundation partner organization vaccines save 25 million children from preventable diseases every year The measles vaccine has decreased childhood deaths from.
. Your Guide to Diabetes. In recent years the COVID-19 pandemic and reckoning with racial injustice has exacerbated an existing mental health crisis. Centers for Disease Control and Prevention.
1 2020 to Sept. COVID-19 vaccine side effects in children and teens. 6m-4yr covid vaccine is now approved and availableYou can schedule to receive the Pfizer or Modarna vaccines at our partner Practice Primary Pediatrics in Castro Valley and only Moderna vaccine at our partner practice in San Leandro by calling.
The COVID-19 vaccine has no live virus. Through the first week of September however 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds the Centers for Disease Control and Prevention reported. Please find the closest location by visiting.
Vincent Iannelli MD is a board-certified pediatrician and fellow of the American Academy of Pediatrics. Updated on December 03 2020. At age 1215 months or 8 weeks after dose 2 whichever is later.
The COVID-19 vaccine does NOT contain ingredients that are known to be harmful to pregnant women or to the fetus. The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases 29 million than children aged 5-11 years 56 million cases and 12-15 30 million cases but more deaths. On Developmental and Behavioral Pediatrics Executive Committee Members.
Adherence to the American Academy of Pediatrics vaccine schedule is a fundamental part of pediatric. COVID-19 Vaccine for Kids Ages 12 to 15. Your COVID Vaccine Card.
The COVID-19 vaccine is our best hope for ending the current pandemic. Cumulatively about 156000 children were hospitalized with COVID from Aug. Centers for Disease Control and Prevention.
Children and COVID-19 vaccination trends. 28 2020 -- The American Academy of Pediatrics recommends that pediatricians take the steps they would for any potential infectious disease outbreak including preparing their offices to adopt standard infection-control practices collaborating with their local hospital and. COVID-19 vaccine and other vaccines may be administered on the same day.
As a result in 1999 the Centers for Disease Control CDC and the American Academy of Pediatrics AAP asked vaccine makers to remove thiomersal from vaccines as quickly as possible on the precautionary principle. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. Versie 6 september 2022 versie 213 Zie onderaan pagina voor een overzicht van de wijzigingen onder versiebeheer.
What You Need to Know. The COVID-19 vaccine will prevent the vast majority of COVID-19 infections. We then recommend yearly visits through adolescence.
Can My Child Test Positive for COVID-19 if They Already Had It. American Academy of Pediatrics 201867111. 5 Things You Should Know.
Covid-19 Vaccine Update June 22nd 2022. As COVID-19 infections go up in our communities your risk of getting COVID-19 goes up too. The Pfizer-BioNTech COVID-19 vaccine was approved by the CDC in persons aged 16 years on December 12 2020 following an EUA from.
The proportion of ED visits with diagnosed COVID rose steadily throughout June and July as 7-day averages went from 26 on June 1 to 63 on July 31 for children aged 0-11 years from 21 to 31 for children aged 12-15 and from 24 to 35 for 16- to 17-year-olds according to data from the Centers for Disease Control and Prevention. Learn about our editorial process. Article Navigation From the American Academy of Pediatrics Clinical Report January.
On December 18 2020 FDA issued an Emergency Use Authorization for Moderna COVID-19 vaccine to prevent. Of zie paragraaf 13 voor een meer uitgebreide toelichting op de wijzigingen in deze versie tov. 2018 report of the Committee on Infectious Diseases.
National Diabetes Information Clearinghouse. COVID-19 vaccine and other vaccines may be administered on the same day. Pro 1 Vaccines can save childrens lives.
Pediatric Covid Vaccine Now available. American Academy of Dermatology Recognizes Dr. There is no evidence among clinical trial participants or the millions who have received the vaccines since authorization that the vaccine.
Additional visits may be necessary if any special needs arise. 548 so far versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds the COVID Data Tracker. 176 A recent JAMA Pediatrics study found that the number of children.
Dit is een nieuwe uitvoeringsrichtlijn COVID-19-vaccinatie versie 2 de opvolger van de vorige versie versie 1. At age 1215 months or 8 weeks after dose 2 whichever is later. Sylvia Hsu with 2021 Mentor of the Year Award.
CDC COVID-19 Response Team Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine United States December 21 2020-January 10 2021 MMWR Morb Mortal Wkly Rep. The CDC and FDA have approved Covid-19 vaccinations for children 6 months through 4 years of ageAs of today we have received the Pfzier Covid-19 vaccinations and are currently scheduling vaccine appointments beginning on June 22 2022. While the goal will be to administer vaccine to as many people as possible as quickly as possible vaccine supply will be limited initially.
What To Know About Modernas COVID-19 Vaccine For Kids 6 and Under. The American Academy of Pediatrics recommends COVID-19 vaccination for all children and adolescents 6 months of age and older who do not have contraindications using a vaccine authorized for use for their age. Section on Developmental and Behavioral Pediatrics Executive Committee 20182019.
Thiomersal is now absent from all common US and European vaccines except for some preparations of influenza vaccine. Children and COVID. Iannelli has cared for children for more than 20 years.
COVID-19 Guidance for Children. Iannelli has cared for children for more than 20 years. 10 2022 according to the CDC which puts the total number of pediatric.
Pfizer COVID-19 Vaccine Approved for. Over deze richtlijn 11 Inleiding. What does that mean for parents who stil.
10 Pediatricians Share How They Discuss Vaccines With. 2018 report of the Committee on Infectious Diseases. Pfizer COVID-19 Vaccine Approved for Teens Age 12-15.
According to the American Academy of Pediatrics the COVID-19 vaccine will not cause infertility or disturb puberty. CDC recommends pediatric COVID-19 vaccine for children 5 to 11 years. American Academy of Pediatrics.
Please call our office at 919-969-9611 to schedule your childs vaccine. American Academy of Pediatrics 201867111. Subsequent appointments occur at 2 4 6 9 12 15 18 24 and 30 months old.
Vincent Iannelli MD is a board-certified pediatrician and fellow of the American Academy of Pediatrics. ED visits and new admissions change course. In December 2020 the Food and Drug Administration FDA issued Emergency Use Authorizations EUAs for the Pfizer-BioNTech COVID-19 BNT162b2 vaccine and the Moderna COVID-19 mRNA-1273 vaccine and the Advisory Committee on Immunization Practices ACIP issued interim recommendations for their use in persons aged 16 years and 18 years.
Checking Your Blood Glucose Type 2 Diabetes Complications. CDC recommends COVID-19 vaccines for young children. Learn more about the American Academy of Pediatrics including our mission leadership and commitment to the optimal health and.
Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
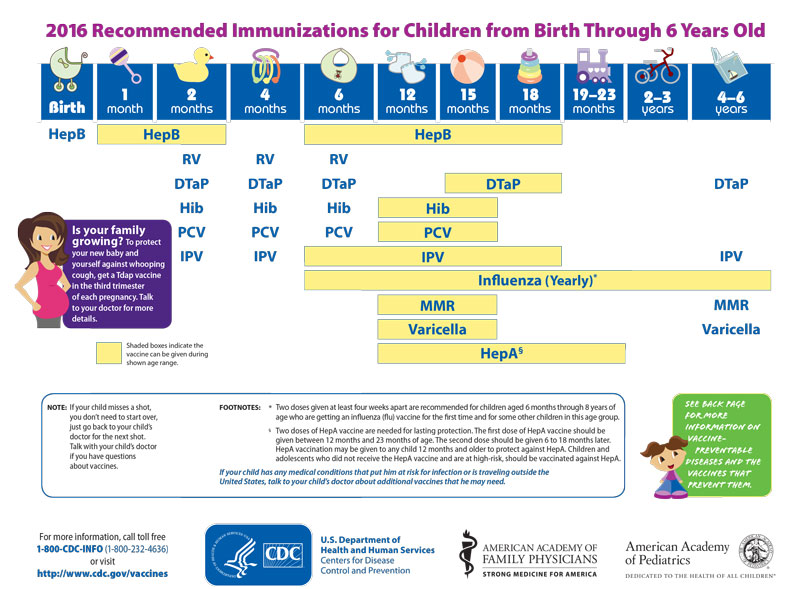
Immunization Schedule 0 5 Yrs Maryland Farms Pediatrics
Pfizer Says Its Covid Vaccine Trial For Kids Ages 12 To 15 Is Fully Enrolled

Covid 19 Vaccine For Kids Benefits Are Many Here Are Answers To Parental Concerns Ask The Pediatrician Column Together Lancasteronline Com
First Covid 19 Vaccine Authorized For Kids Ages 12 To 15 Smart News Smithsonian Magazine

Pediatrician Answers Your Questions On Pfizer Covid 19 Booster For Ages 12 15 Cnn
What Do We Know About Covid 19 In Kids Now Whyy

Children 12 15 Years Old Can Now Get The Pfizer Covid 19 Vaccine Uconn Today

Covid 19 Vaccine For 12 To 15 Year Olds What Parents Should Know Methodist Health System Omaha Council Bluffs Fremont

Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
Kids Covid Vaccine Side Effects What To Know
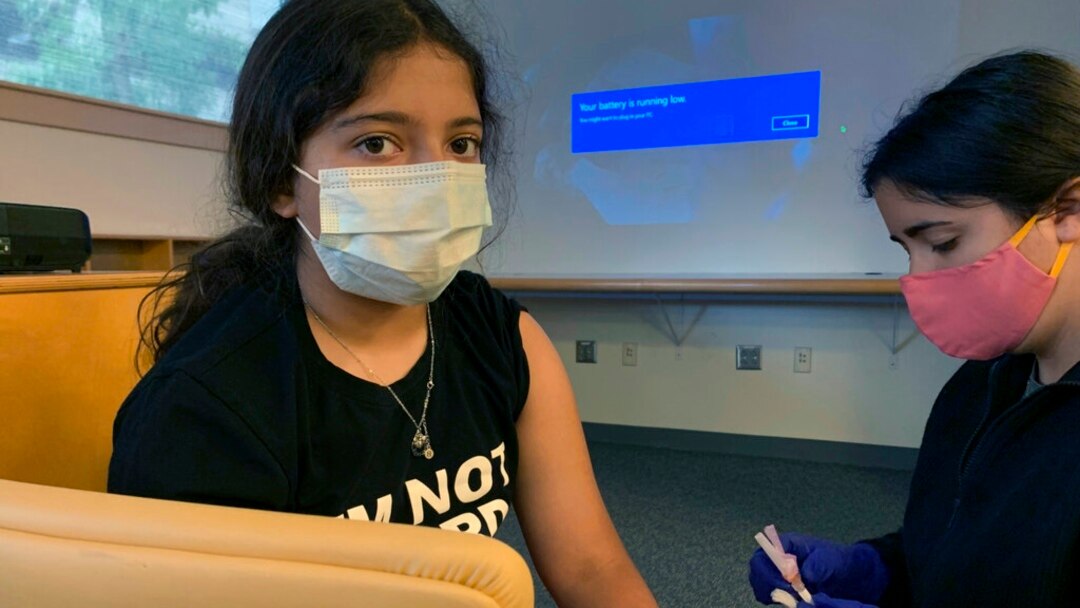
Drugmakers Pfizer Covid 19 Vaccine Safe For Children Ages 5 11
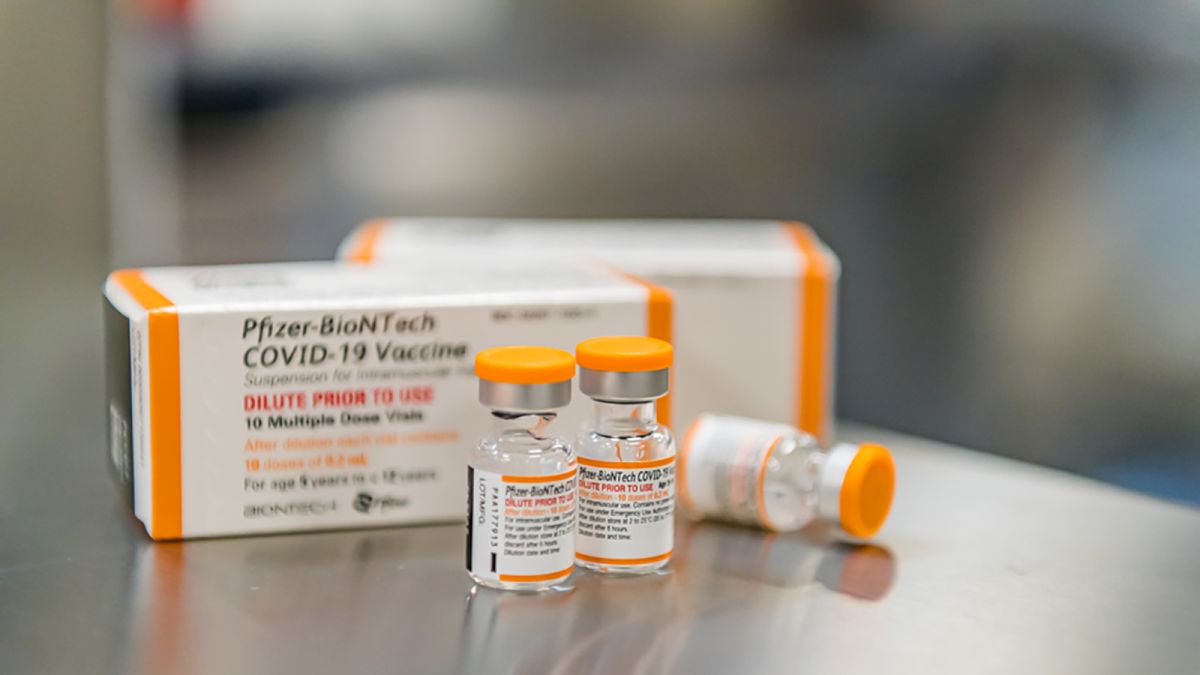
Younger Kids Could Be Getting A Covid 19 Vaccine Within Weeks Cnn
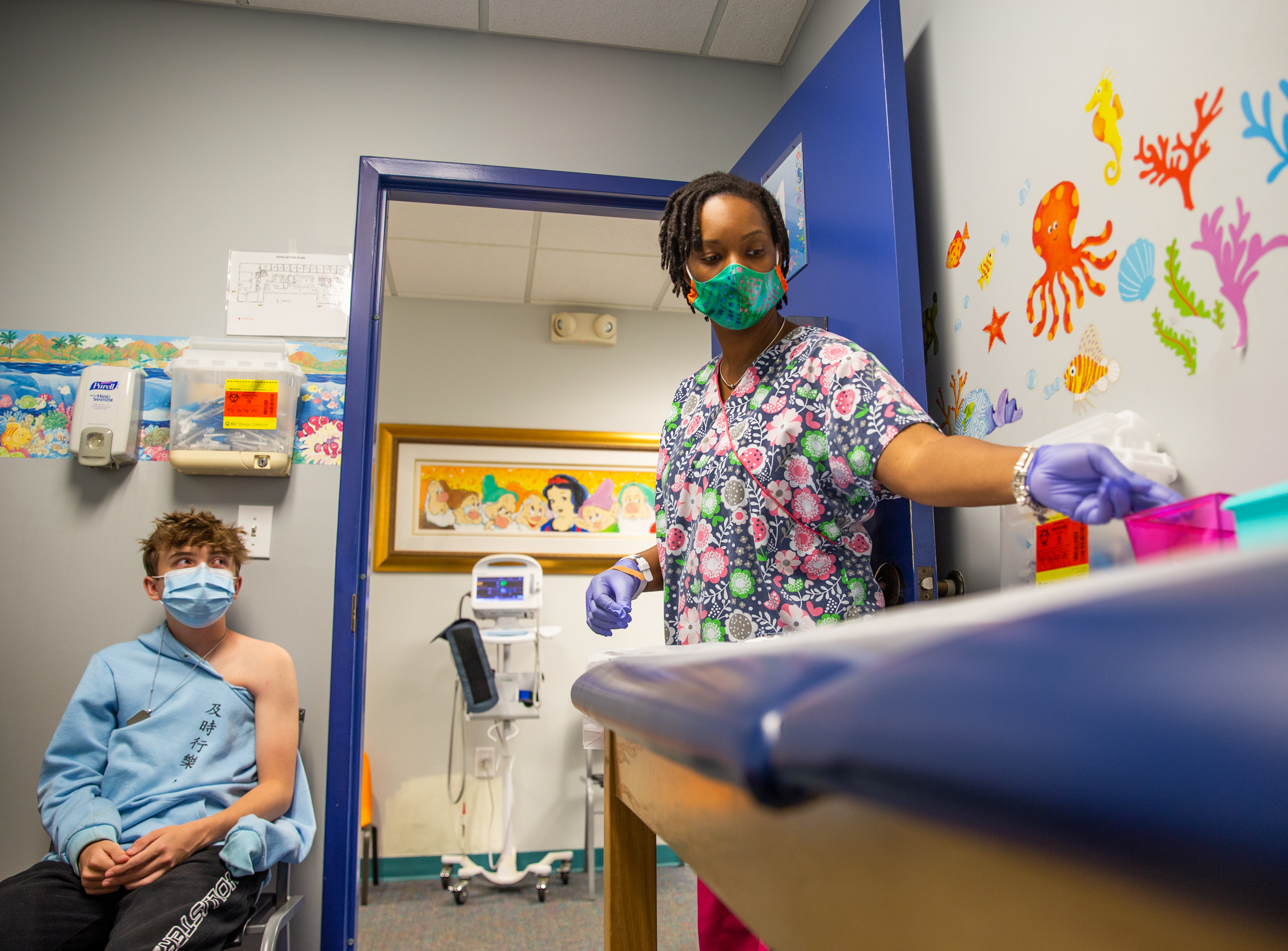
Fda Authorizes Pfizer Covid Vaccine For Children 12 To 15

An Expert Explains Are Covid 19 Vaccines Safe For Children World Economic Forum
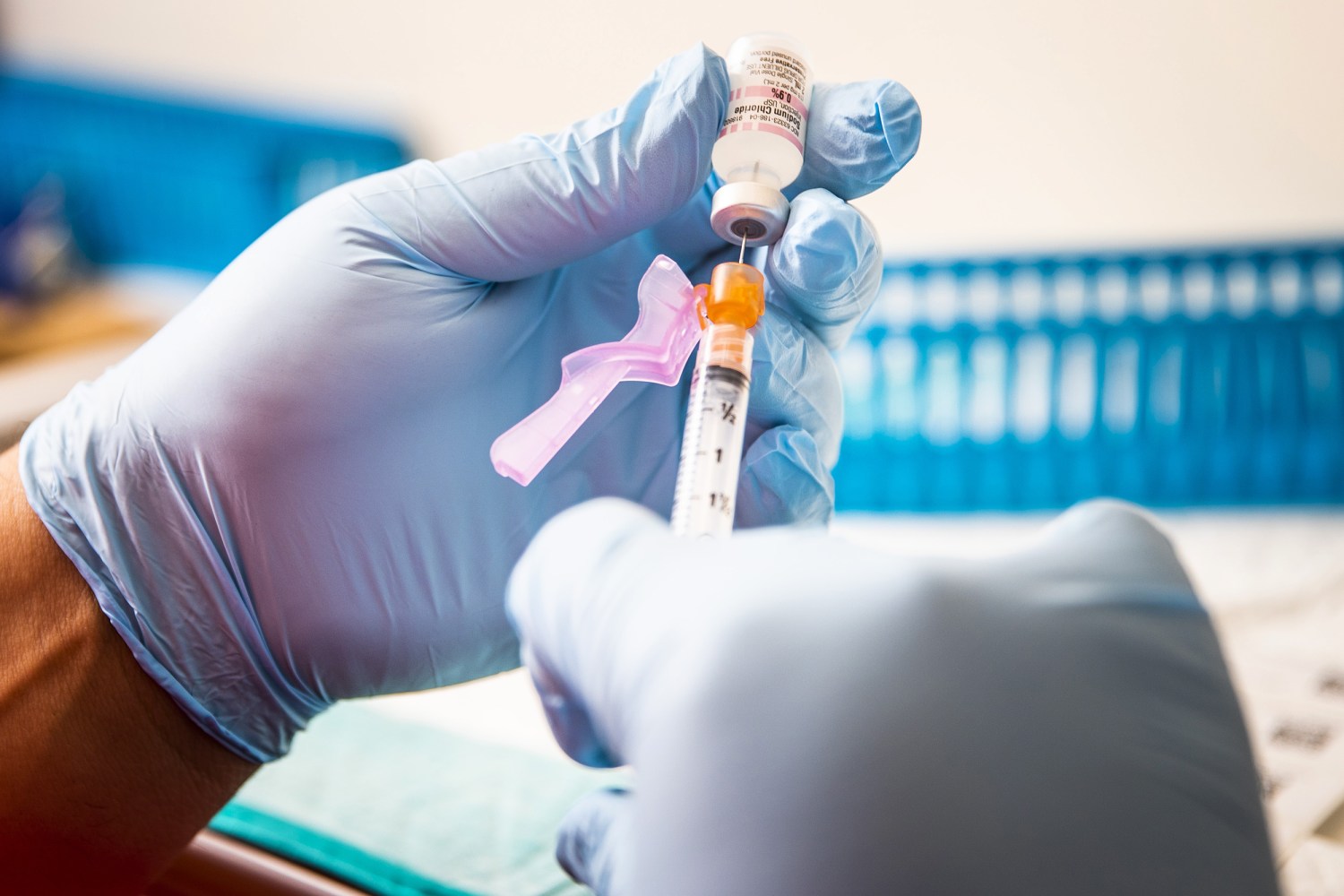
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says